eBooks View all | By AtoZ | By category
The Hops Tethering Complex and the Dynamin Homolog Vps1 Coordinate Distinct Stages in Intracellular Membrane Fusion
By Aditya Kulkarni
UT Southwestern Medical Center, USA
Published: Oct 17, 2016 | pg. no: 1-71
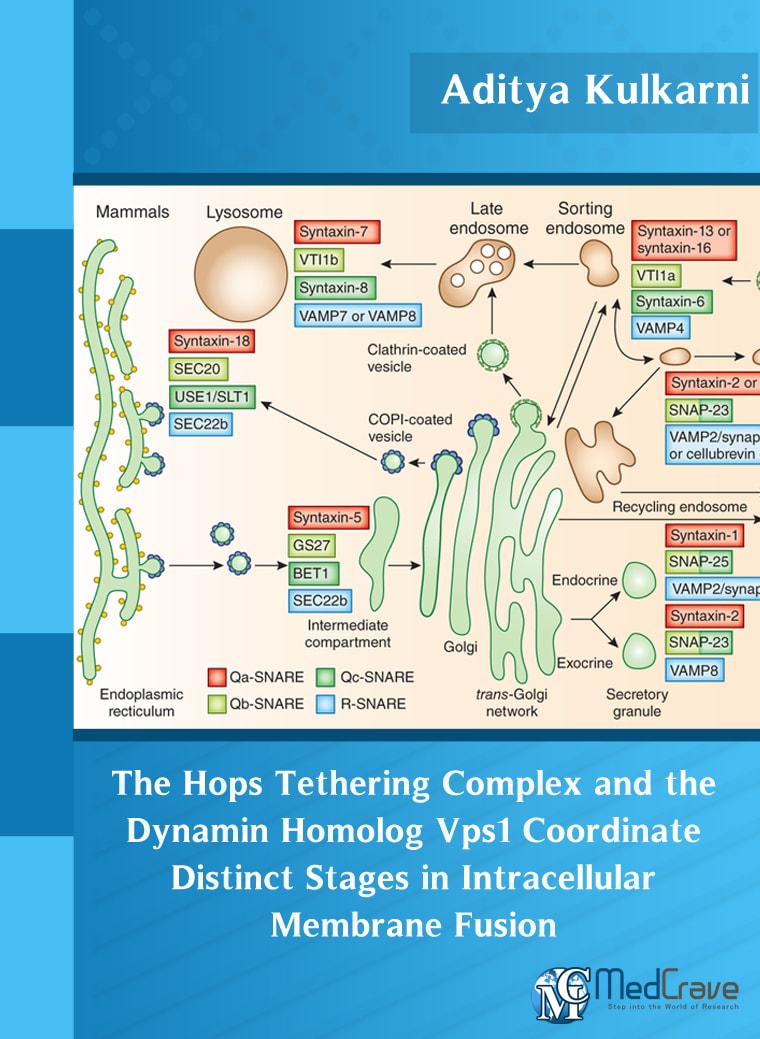
Abstract: Cells in almost all eukaryotes ranging from yeast to humans contain membrane-enclosed compartments called organelles. To survive and proliferate, cells must communicate signals within themselves and with their environment and this is achieved through membrane trafficking. It involves generation of a vesicle carrying specific soluble or membrane-anchored cargo from a precursor membrane, transport of the vesicle to its destination and ultimately fusion of the vesicle with the target membrane. Soluble N-ethylmaleimide sensitive factor Attachment protein Receptors (SNAREs) have been identified as key components of membrane fusion in the endomembrane system. SNARE proteins typically consist of a single transmembrane domain at the carboxy terminal and a coiled-coil SNARE domain and are designated as Qa, Qb, Qc and R depending upon the presence of a Glutamine or Arginine residue at a conserved position in the SNARE domain. A critical intermediate in the membrane fusion pathway is the trans-SNARE complex generated by the assembly of SNAREs residing in opposing membrane compartments destined to fuse. Mechanistic details of trans-SNARE complex formation and topology in a physiological system remain largely unresolved. This study on native yeast vacuoles revealed that SNAREs alone are insufficient to form trans-SNARE complexes and that additional factors, potentially tethering complexes and Rab GTPases, are required for the process. HOmotypic fusion and vacuole Protein Sorting (HOPS) is a tethering complex that is known to function in endosome to vacuole/lysosome transport and is well conserved across multiple species. Ypt7 is the Rab7 homolog in yeast that coordinates endolysosomal trafficking. I found that the HOPS complex exists as a dimer on the surface of yeast vacuoles. I report a new finding that a HOPS tethering complex dimer catalyzes Ypt7-dependent formation of a topologically preferred QbQcR-Qa trans-SNARE complex in yeast vacuole fusion.
View eBook